Early Phase I Window of Opportunity Biomarker and Safety Trial to Test Pyrimethamine as an Inhibitor of NRF2 in HPV-negative, Locally Advanced Head and Neck Squamous Cell Carcinoma
NRF2 activation, observed in up to 40% of head and neck squamous cell carcinoma (HNSCC) tumors, plays a critical role in tumor progression, metastasis, and radiation therapy resistance. We have recently discovered that pyrimethamine (PYR) and its analogs have an inhibitory effect on NRF2 activity in vitro and in mouse models via inhibition of dihydrofolate reductase (DHFR).
Pyrimethamine is an established drug that has been used for decades for treatment of protozoan infections and malaria. A growing body of research shows that it has potential antitumor activity, however its activity on growing human tumors has not been previously studied. The primary efficacy goal of this study is to evaluate the activity of pyrimethamine on human tumors as demonstrated by inhibition of DHFR and downregulation of NRF2 pathway activity. On-target inhibition of DHFR by pyrimethamine results in the stabilization and increased protein expression of human DHFR.
The primary efficacy hypothesis of this study is that treatment with pyrimethamine will result in a 50% increase in DHFR protein within the tumor cells as measured by quantitative western blot analysis. Secondarily, among those tumors classified as NRF2-active on pre-treatment biopsy, we hypothesize there will be a 50% reduction in NRF2 activity as measured by SureQuant targeted proteomic analysis.
Primary Objectives:
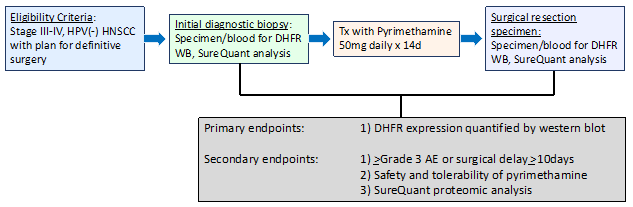
To determine the change in tumor DHFR expression following neoadjuvant treatment with pyrimethamine.
Secondary Objectives:
1. To determine the proportion of patients who experience ≥ grade 3 AE due to pyrimethamine or delay in surgery by ≥ 10 days due to pyrimethamine.
2. To establish the safety and tolerability of neoadjuvant treatment with pyrimethamine.
3. To determine the change in NRF2 pathway activity following neoadjuvant treatment with pyrimethamine.
Primary Efficacy Endpoint:
Log2 of tumor DHFR expression using quantitative western blot analysis in post-treatment sample relative to pre-treatment.
Secondary Endpoints:
1. Occurrence of ≥ grade 3 AE due to pyrimethamine or delay in surgery by ≥ 10 days due to pyrimethamine.
2. Safety and tolerability by CTCAE v 5.0.
3. Log2 of NRF2 activity score using the NRF2 SureQuant proteomic assay in post-treatment sample relative to pre-treatment
This study will enroll 20 evaluable patients with treatment-naïve HPV-negative, locally advanced head and neck squamous cell carcinoma with a treatment plan that includes primary surgical resection.